Which of the Following Correctly Describe Atomic Structure
The bond angles around C1 will be equal to 90. Neutrons and electrons are found in space as a cloud around the nucleus.

Atomic Structure And Subatomic Particles Youtube
Choose one correct answer in each drop-down list 1 1 8.

. Protons and neutrons have approximately the same mass about 167 10-24 grams which scientists define as one atomic mass unit amu or. An atom has more electrons than protonsC. Select all that apply-The nucleus compromises a very small fraction of the total volume of an atom-The number of protons equals the number of electrons in a neutral atom-The protons and neutrons together comprise most of the mass of the atom.
Check all that apply. Match each particle or set of particles with the correct description Protons and neutrons Comprise essentially all of the mass of an atom Electrons and protons The subatomic particles that bear Coulombic charges Electrons Reside outside the nucleus of the atom Neutrons Do not bear Coulombic charges 10. The electrons are outside the nucleus and have a negative charge.
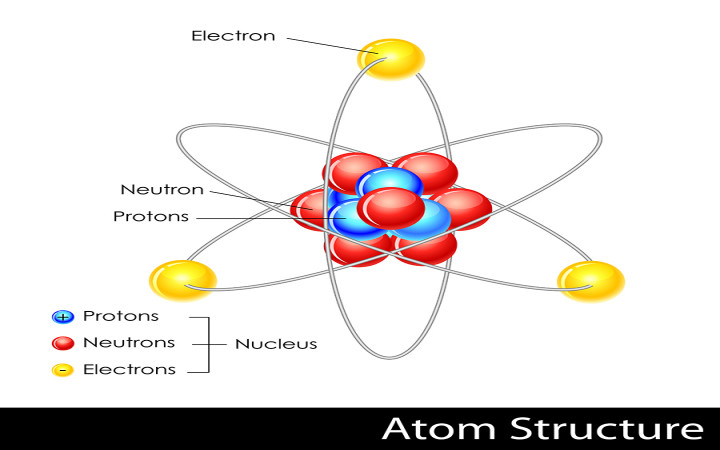
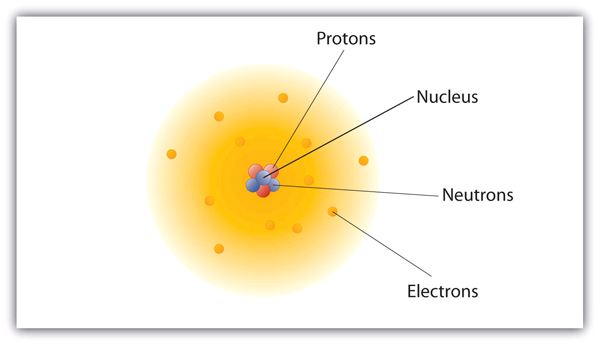
The nucleus which is in the center of the atom and contains protons and neutrons and the outer region of the atom which holds its electrons in orbit around the nucleus. Locate the atomic number in a periodic table of elements. Name and describe the structure of atoms including its mass and locations of protons neutrons and electrons inside an atom.
Which of the following statements correctly describe an atomic orbital-an atomic orbital describes the path of an electron orbiting an atom-an atomic orbital defines the probability of finding an electron in a given region of an atom-an atomic orbital is the square of a Schroedinger wave function-an atomic orbital is a Schroedinger wave function. Atomic structure refers to the structure of an atom comprising a nucleus centre in which the protons positively charged and neutrons neutral are present. Which of the following is the furthest from the center of an atomProtonsNeutronsElectrons Nucleus Which of the following best describes an atoma.
Which of the following options correctly describe the structure shown. The subatomic particles of an element retain the properties of that element. Which of these statements correctly describe the atom.
OElectrons can occupy any possible quantum state in an atom. Repulsion between closely packed protons in the nucleus is overcome by 1 1 1. CHM 2045 S22 Test 3 Form B 5.
The history of atomic structure and quantum mechanics dates back to the times of Democritus the man who first proposed that. An atom is composed of two regions. The electrons are outside the nucleus and have no charge.
Electrons are present outside the nucleus. Identify the subatomic particles associated with mass number. This information indicates that Aatomic number Batomic mass Chalf-life Dmolar.
The bond angles around C2 are approximately equal to 180. Protons and electrons are grouped together in an alternating patternc. C2 will have a linear geometry.
Select all that apply. Which statement correctly describes the location and charge of the electrons in an atom. Electron- the Negative charge which is present outside the nucleus.
A The nitrite ion contains two bonds that are equivalent. The negatively charged particles called electrons revolve around the centre of the nucleus. An atomic nucleus contains protons and neutronsB.
The exclusion principle states that only one electron can exist in each quantum state of an atom. An Oxygen O atom contains 8 protons inside the nucleus and 8 neutrons outside the nucleus. Select all that apply.
The correct answer is B. All matter on Earth is made up of atoms. An Aluminum Al atom contains 27 electrons and 27 protons inside the nucleus.
The structure of an atom is defined as follows-Nucleus - the heavy mass present in the center of an atom. Draw the Lewis structure for the nitrate ion NO 2 including any valid resonance structures. Which of the following statements are correct.
C1 will have a tetrahedral geometry. Which of the following statements correctly describe the electron cloud of the atom. Which of the following statements concerning atomic structure isare correct.
Which of the following correctly describes the atomic structure. Protons and electrons are grouped together in a random patternb. The electron is negatively charged and is located in a cloud surrounding the nucleus.
The overall geometry of this molecule is octahedral. According to the nuclear model the atom is described as having a. Which of the following statements correctly describe the electron cloud of the atom.
Proton - the positive charge which is present inside the nucleus. Repulsion between closely packed protons in the nucleus is overcome by 1 1 9. The neutron has no charge and identifies the element.
The electrons are inside the nucleus and have no charge. Identify the electric charge of an atom and its subatomic particles. Bmore isotopes have an atomic mass of 2 or 3 than of 1 Cmore isotopes have an atomic mass of 1 than of 2 or 3 Disotopes have only an atomic mass of 1 33Hydrogen has three isotopes with mass numbers of 1 2 and 3 and has an average atomic mass of 100794 amu.
A Beryllium Be atom contains 4 electrons outside the nucleus and 5 neutrons inside the nucleus. D Question 8 333 pts Which of the following correctly describe the principles that govern the atomic structure of a multielectron atom. The nucleus is surrounded by a double membraneD.
A core of protons and neutrons surrounded by electrons Which student correctly. Which of the following correctly describes the resonance hybrid actual structure of NO 2. The nucleus contains all the positive charge of an atom.
The number of neutrons determines whether the nucleus of an atom. Place the atomic particles in order from the first one discovered to the last one discovered. It revolves around it.
All atoms of the same element have the same number of neutronsE. Choose one correct answer in each drop-down list 1 1 8.
What Is Inside An Atom Wonderopolis

Atomic Structure Periodic Table Science Quiz Quizizz

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science

Atomic Structure Electrons Protons Neutrons And Atomic Models

Lesson Explainer Modern Atomic Theory Nagwa

Atomic Models Thomson S Atomic Model And Rutherford S Atomic Model
Atomic Theory Introductory Chemistry 1st Canadian Edition
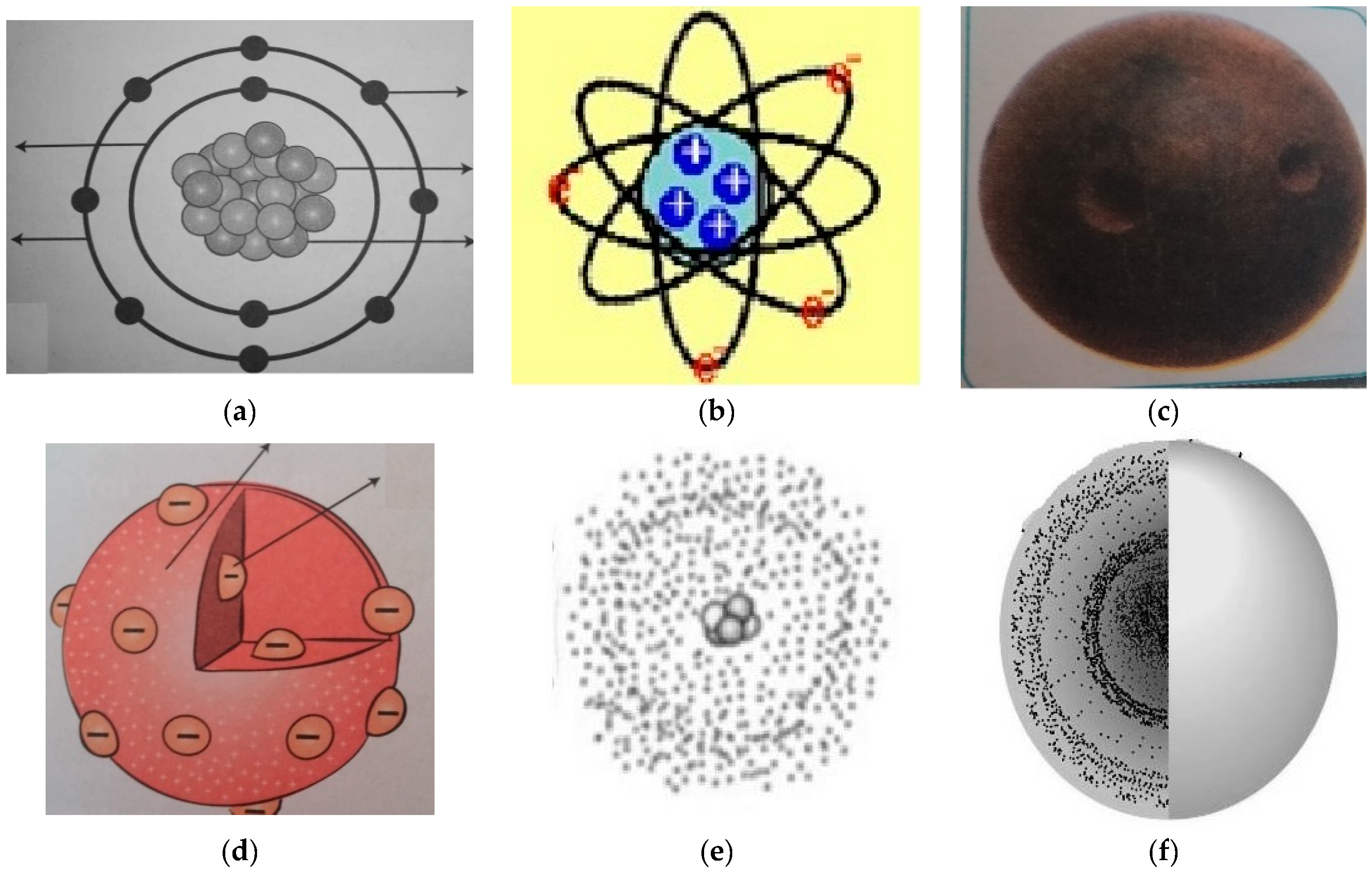
Education Sciences Free Full Text Insights Into Components Of Prospective Science Teachers Mental Models And Their Preferred Visual Representations Of Atoms Html

The Bohr Model Introduction To Chemistry
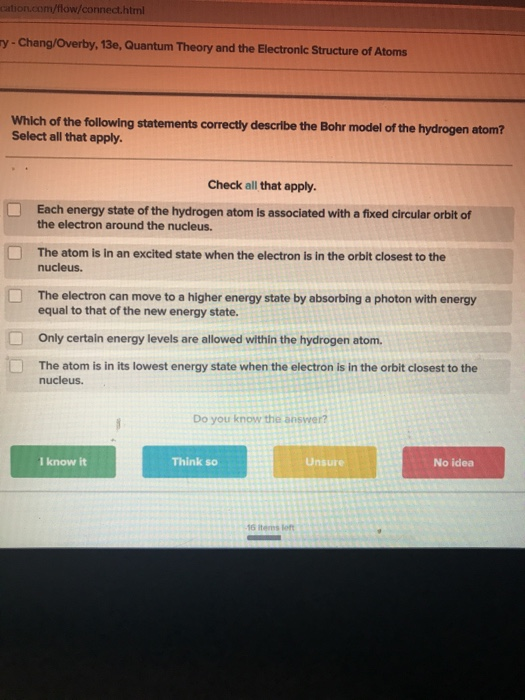
Solved Sation Com Flow Connect Html Chang Overby 130 Chegg Com

Atomic Structure Electrons Protons Neutrons And Atomic Models
What Is The Atomic Structure Of Helium Quora
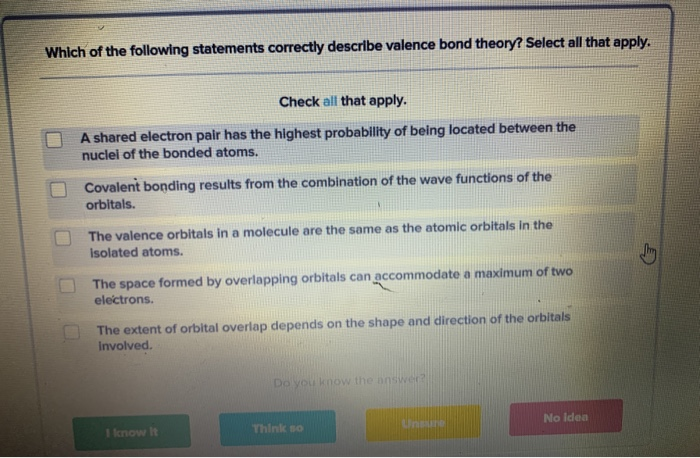
Solved Which Of The Following Statements Correctly Describe Chegg Com
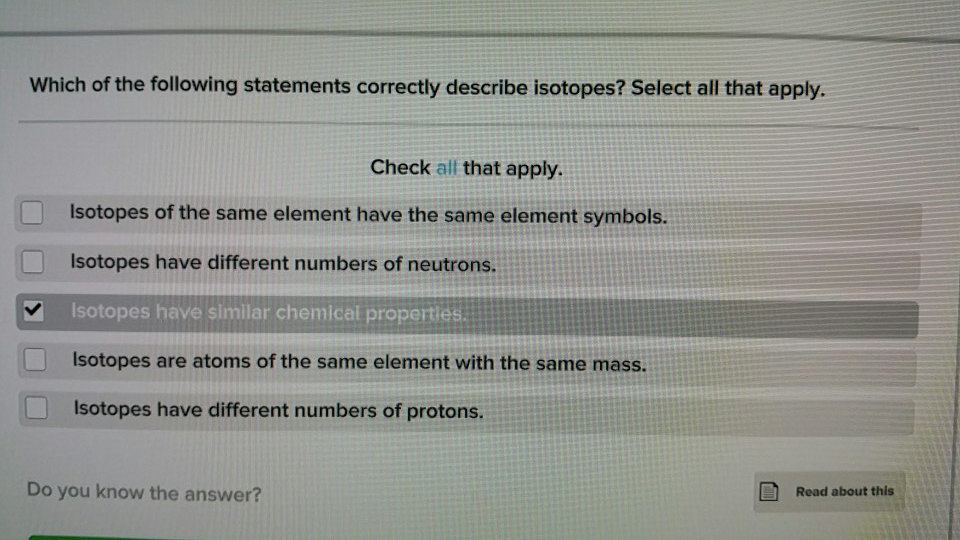
Solved Which Of The Following Statements Correctly Describe Chegg Com

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science



Comments
Post a Comment